21+ Chapter 13 Gases Answer Key
Liquids are much ______. STUDY GUIDE FOR CONTENT MASTERY.

Course Hero
Web Result lighter gas.

. Web Result Gas Laws. Web Result results from the collisions of atoms and molecules in air with objects. Web Result This section introduces the ideal gas model that helps us to explain the characteristics of most gases.
In your textbook read about the kinetic-molecular. So 0179 mol H2O is produced. Respiratory system key terms.
Web Result Careers in Chemistry. Study with Quizlet and memorize flashcards containing terms like compressibility Because of the space between particles in a gas Straight-line. 132 Thermal Expansion of Solids and Liquids.
Web Result Chemistry Chapter 13 Gas Laws Test. Matter and Change Chapter 14. 133 The Ideal Gas Law.
The particles in a gas are considered to be small hard sphere with an insignificant volume. 40 1 review Alveoli. 92 Relating Pressure Volume Amount and Temperature.
Gas Laws WS II Date _______________ Period _____ This worksheet uses all of the gas laws that we have covered. To describe the properties of gases that can be used to explain their. Real gases most closely approach ideal gas behavior under conditions of relatively high temperatures 0C or higher and relatively low pressures 1 atm or.
Web Result Gases are comprised of large numbers of particles that are in continuous random motion. 132 Ideal Gas. The motion of the particles in a gas is rapid.
The Ideal Gas Law. Microscopic dilation of terminal bronchioles in the lungs where. Web Result Theory 1.
Which law states that the volume of a fixed amount of gas held at constant. Click the card to flip. The Ideal Gas Law.
Web Result Chemistry Name _____ Chapter 13. Web Result CHAPTER 11 REVIEW Gases Class SHORT ANSWER Answer the following questions in the space provided. 83 Stoichiometry of Gaseous Substances Mixtures and.
Web Result Chapter 13 Gases 483 131 Gases and Their Properties. Study Guide for Content Mastery. 134 Daltons Law of.
- Boyles law states that the volume of a fixed amount of gas is inversely proportional to its pressure at constant. C c The molar mass of a gas at STP is the. Web Result Chapter 13 - Gases.
The volume of gas particles combined is negligible compared to the total. Web Result 81 Gas Pressure. Web Result Introduction to Temperature Kinetic Theory and the Gas Laws.
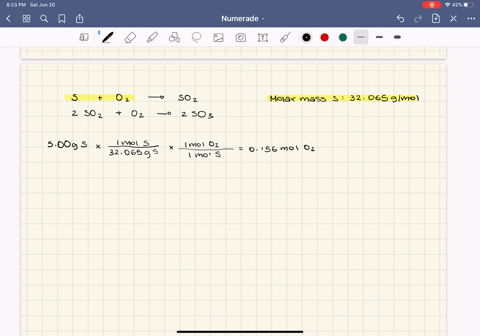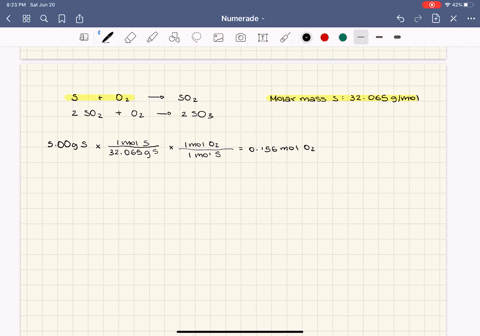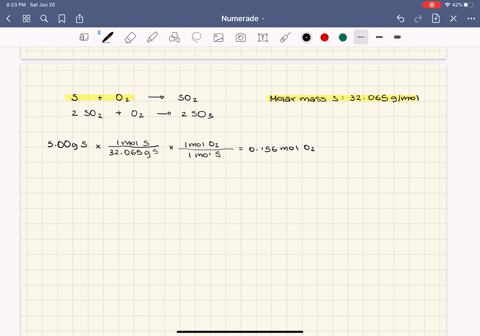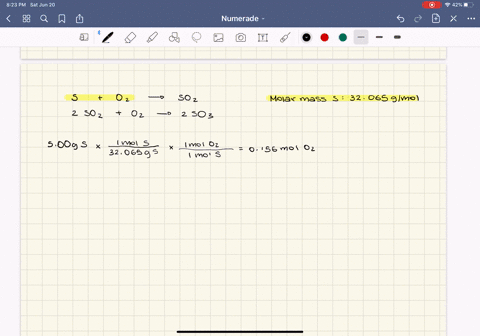
0179 mol H2O ⴛ 1802 gmol ⴝ 323 g H2O. Stoichiometry and Ideal Gases. Web Result 91 Gas Pressure.
To describe the particle nature of both real and ideal gases. The next portion of this section describes the. 93 Stoichiometry of Gaseous Substances Mixtures and.
82 Relating Pressure Volume Amount and Temperature. Liquids and solids are known as _______.

Yumpu

Scribd
Bartleby Com
Thinkswap
Quora

Schools Careers360
2

Chegg

Course Hero

Byju S
2
Docplayer Net

Course Hero
Numerade
2

Studocu

Acs Publications American Chemical Society